On the Pill? This Brand Was Just Recalled Because of a Packaging Error
Anyone taking Mibelas 24 Fe needs to needs to read this now.


• A packaging error caused packs of Mibelas 24 Fe oral contraceptives to be recalled.
• The recalled birth control pills come from Lot No. L600518 and are marked with an expiration date of 5/31/2018.
• No adverse events or unwanted pregnancies have been reported yet.
After a consumer noticed packaging mistake in one of its oral contraceptives, Lupin Pharmaceuticals voluntarily recalled their Mibelas 24 Fe tablets, according to the FDA.
The packaging error which accidentally rotated the blister pack 180 degrees placed the placebo pills at the beginning of the pack instead of at the end, which is kind of a big deal considering you can get pregnant if you take the pills out of order.
RECALL ALERT: Mibelas Birth Control Tablets Recalled https://t.co/YsTb0FJaNe pic.twitter.com/Tu28BHwwD7June 8, 2017
While the Mibelas birth control was distributed nationwide to pharmacies, clinics and wholesalers, no unwanted pregnancies have been reported yet.
The FDA also points out that unwanted pregnancies aren't the only adverse effect of this packaging error, since unintended pregnancies can be fatal to some women or be harmful to the fetus if the woman is taking other medication that causes birth defects.
Get exclusive access to fashion and beauty trends, hot-off-the-press celebrity news, and more.
RELATED STORIES
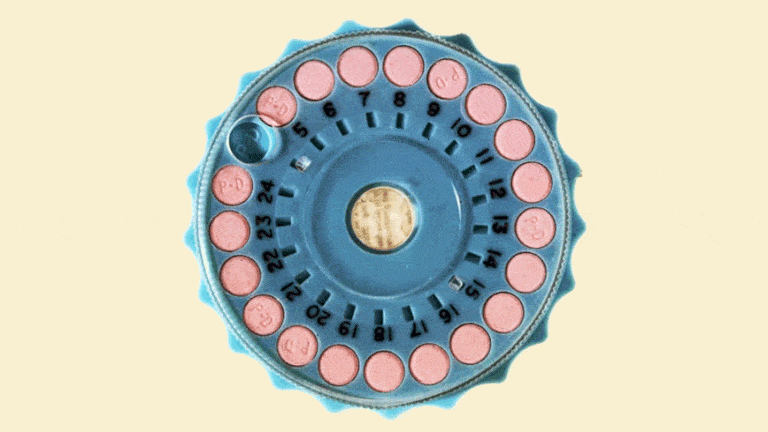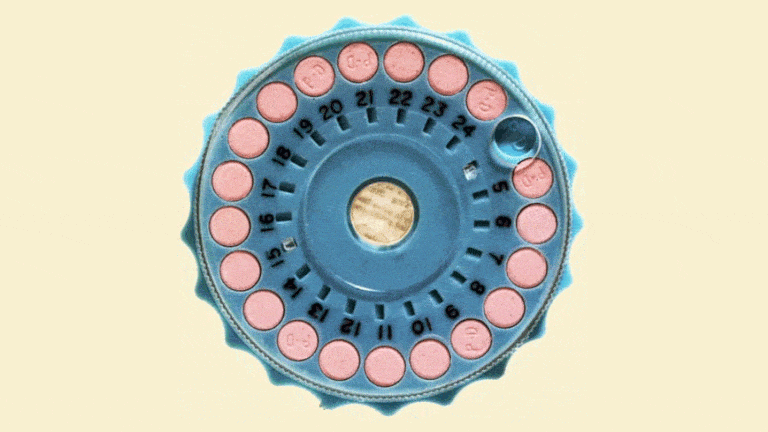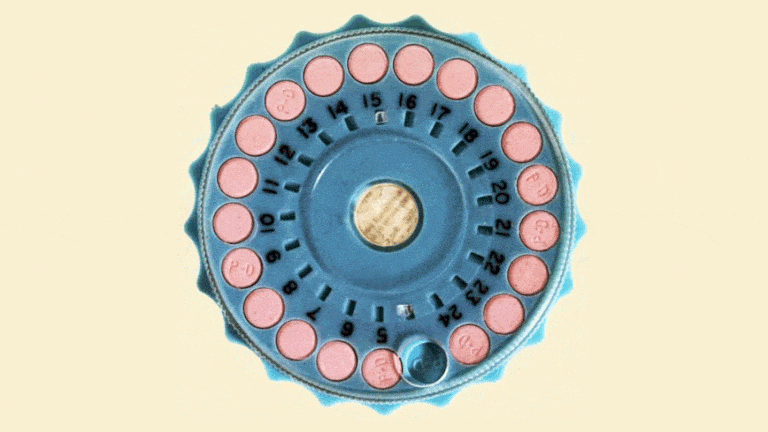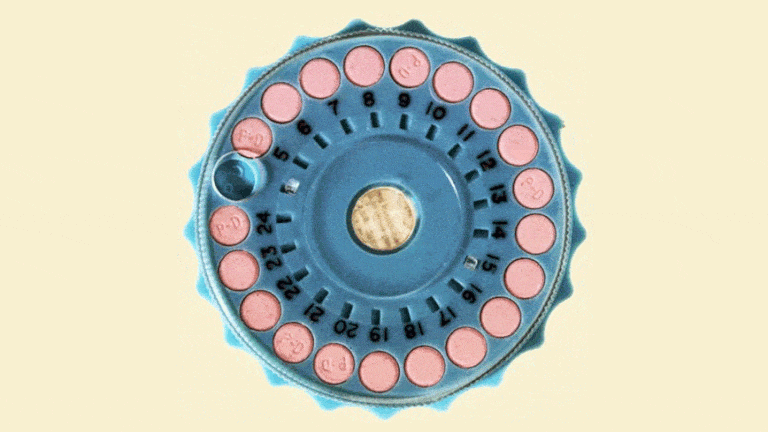


The Mibelas 24 Fe tablets that are being recalled come in blister packs containing 28 tablets, including 24 white pills with the active ingredients debossed with "LU" on one side and "N81" on the other and 4 placebo pills debossed with "LU" on one side and "M22" on the other.
The National Drug Code (NDC) on the individual packs is 68180-911-11 and the NDC for the three-wallet packs is 68180-911-13. Both types come from Lot No. L600518 with the expiration date 5/31/2018.
Contact your doctor or healthcare provider and return the pills to your pharmacy if you have one of these packs of birth control. If you have any questions about the recall, Lupin can be contacted by calling 1-800-399-2561, 8 a.m. to 5 p.m. EST, Monday through Friday.
Follow Marie Claire on Facebook for the latest celeb news, beauty tips, fascinating reads, livestream video, and more.
Lyndsey Matthews is the Destination News Editor for AFAR; previously she was a Lifestyle Editor across all of Hearst Digital Media's brands, and a digital editor at Martha Stewart Weddings and Travel + Leisure.
